
In this post, we show how an exapmple of how liquids can be exchanged and mixed inside the Nano Channel Chip, using fluorescent dye to directly visualize how this happens in the chip.
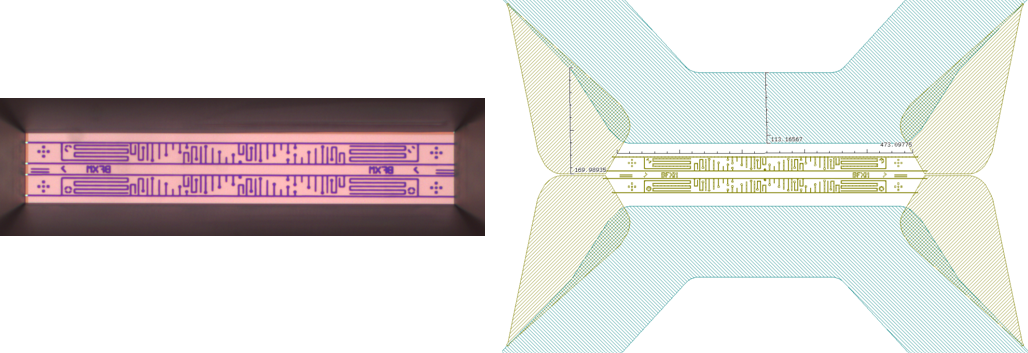
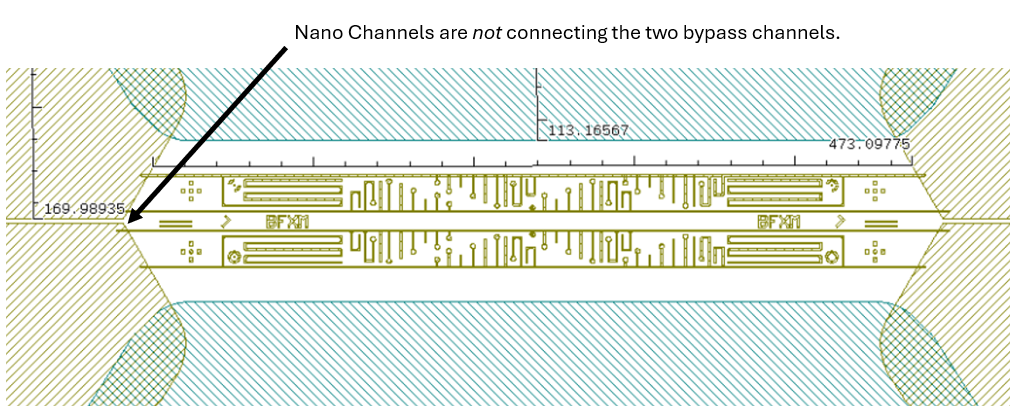
In the experiment, we use the Butterfly X-mas chip, where there are two types of nano channels in the field of view:
The chip is made for the purpose of first filling of dead-end channels with one liquid, via the bypass channel, and then exchanging the liquid in the bypass channel. The second liquid will then diffuse together with the first liquid stored in the dead-end channels, which act like a reservoir.
This is a reliable way of mixing, which ensures that the mixing event actually takes place in the field of view.
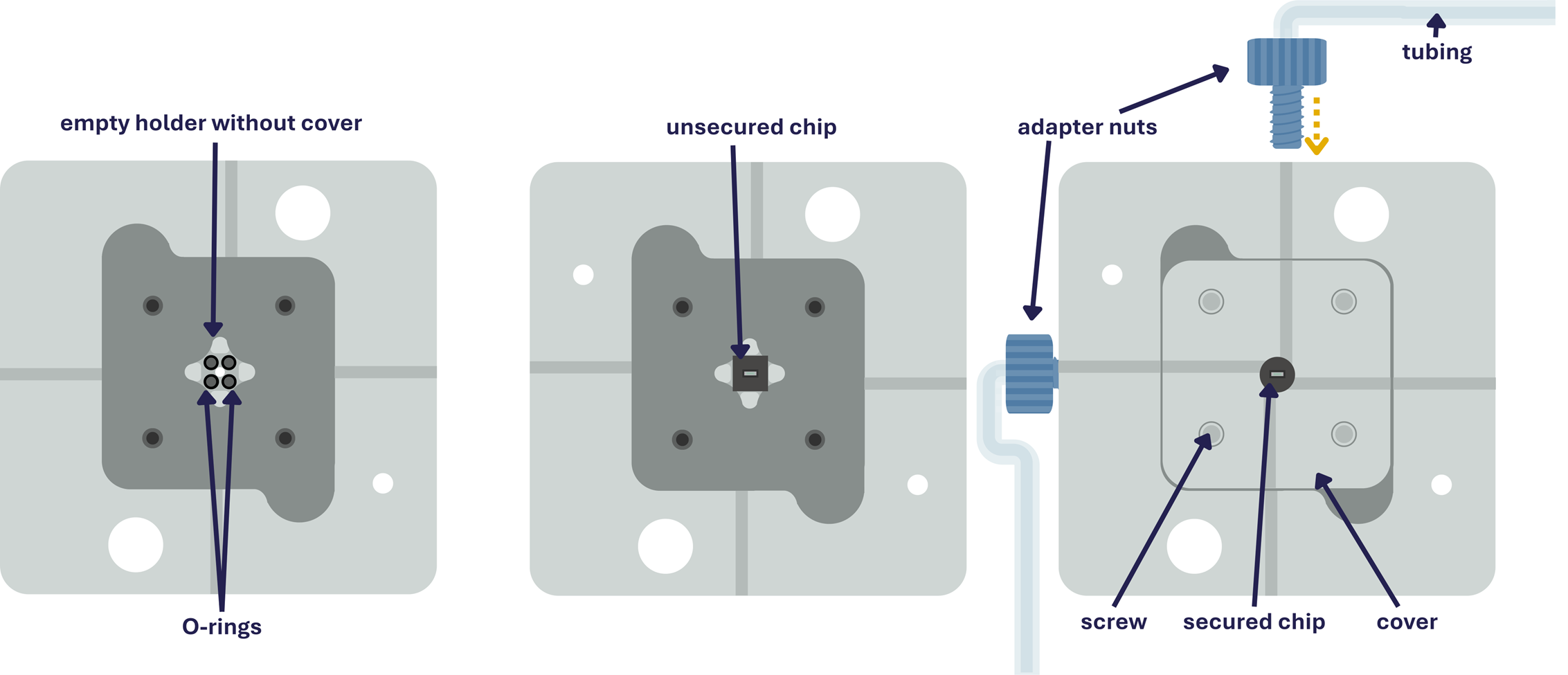
The following components are needed in this experiment, and sketches explain the chip- and holder setup below.



(Small advertisement: This holder can be ordered directly from our website, here: https://www.insightchips.com/e-shop )
A 10 mM Rhodamine B solution was prepared.
For the chip, the protective membranes on two inlets on all sides were punctured. Because the membrane is only a few nanometers thick, a gentle poke with a syringe needle is sufficient to break it.
The Rhodamine solution was added to an inlet in the chip, and capillary forces ensure that the liquid fills the entire inside of the chip.

The other two main channels connecting the second pair of inlets stayed empty. This was confirmed by the color change visible under the optical microscope.


The chip was placed in the flow holder on top of the four O-rings with the inlets facing downward and secured with the cover lid tightened by screws.
Tubing was attached to the holder with adapter nuts, connecting a syringe filled with deionized water to one inlet and an output vial to the other.
Water was then flushed through the chip and nano channels at a rate of 0.20 mL/min using a syringe pump.
The entire flushing process was observed and recorded on an Olympus BX61 fluorescence microscope equipped with a TRITC filter set.
Imaging and camera parameters, such as exposure rate and gain were optimized for best image contrast. Rhodamine B appeared bright red.
The whole setup is sketched here:

The video below shows that fluorescent Rhodamine B clears first from the bypassing nano channels, as expected. Subsequently, the dead-end channels slowly lose intensity as the Rhodamine diffuses out.
Note that the shorter dead-end channels lose all their intensity rather quickly, while the much longer dead-ends stay fluorescent for a much longer time.
Note that the video is sped up 50X. To watch the real-time video, go here: https://vimeo.com/1134116093?fl=ip&fe=ec
Fluorescence microscopy using Insight Chips Nano Channel Chips enables studies of fluid dynamics, such as flow behavior and diffusion at the micro- and nanoscale. Fluorescent dyes allow us to track liquid motion without adding particles, which is useful for characterizing nano channel flow. This serves as an easy method to study flow that can be more difficult to characterize using Electron Microscopy.
As the same Nano Channel Chips can be used for both optical and electron microscopy, the flow behavior knowledge and understanding can be utilized to conduct more informed electron microscopy experiments, so as to obtain higher resolution characterization in liquids.
With the SEM/Optical Flow Holder, the Nano Channel Chip chip can be connected to flow control systems with a simple setup. Providing more advanced and precise regulation and control of pressure and flow for more complex experiments.